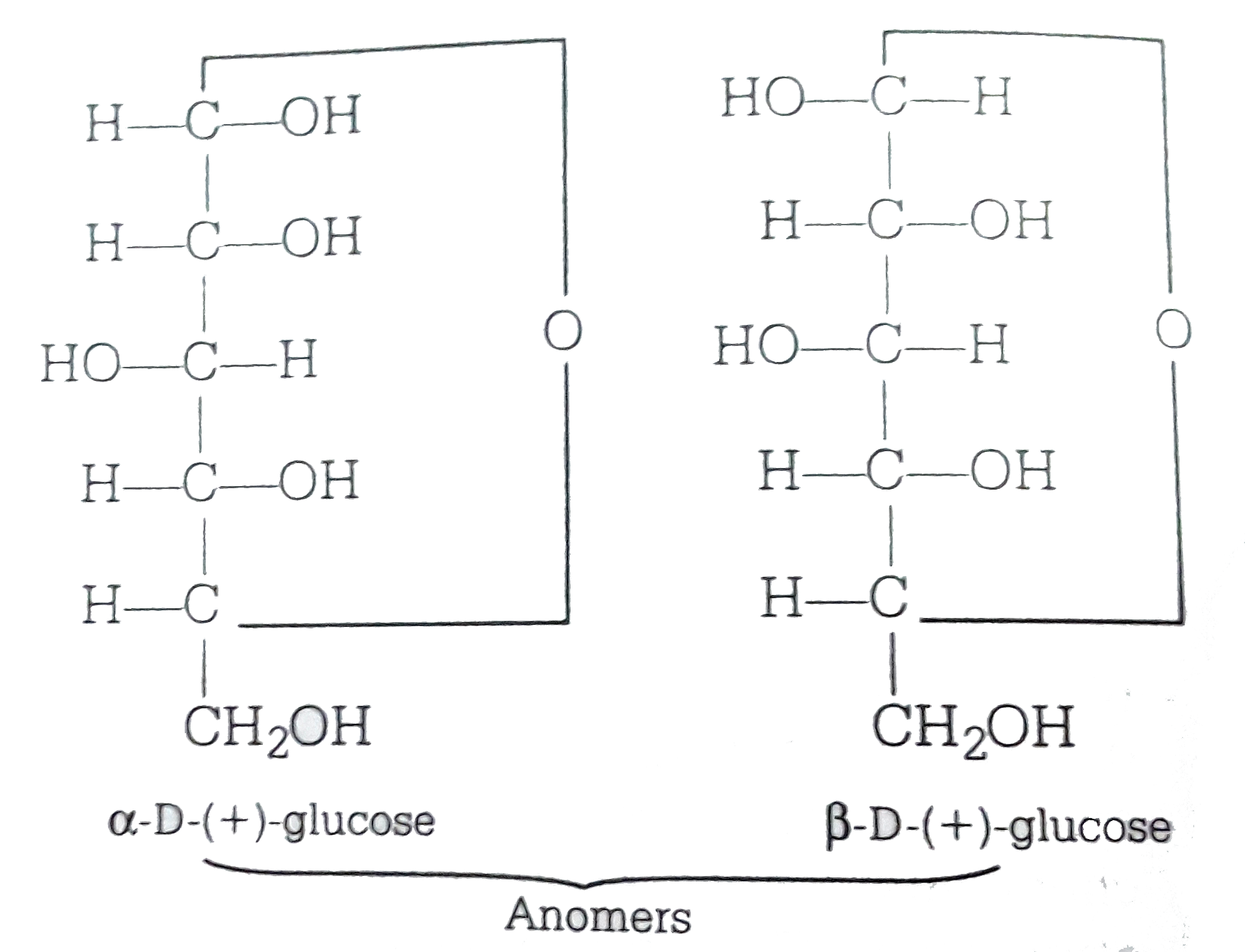
Alpha D Glucose and Beta D Glucose Are Enantiomers
For example, let's consider the glucose molecule in its open-chain form (recall that many sugar molecules can exist in either an open-chain or a cyclic form). There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore.

Enantiomers Of Alpha D Glucose Johnathon Howells
D- and L-is an old but still-convenient shorthand for saying that molecules are enantiomers. e.g. D-glucose and L-glucose are non-superimposable mirror images without having to write out a long IUPAC name with lots of ( R) and ( S) descriptors. Most natural sugars are D- and most natural amino acids are L- .
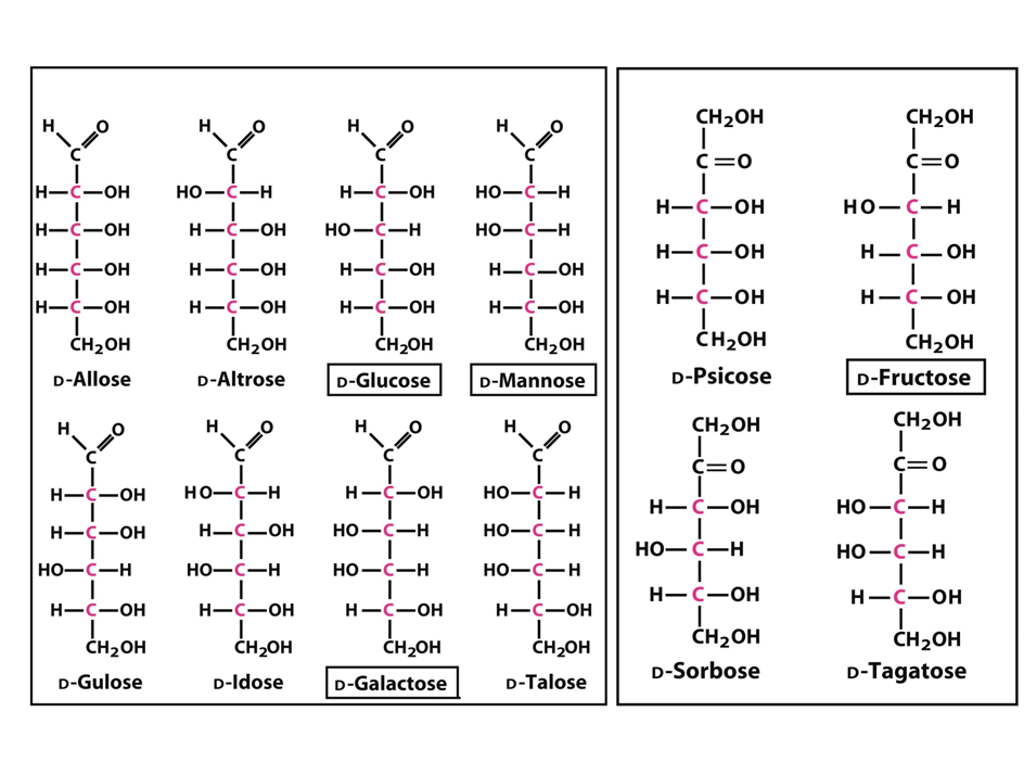
Solved CH2OH CH2OH HCOH HCOH HCOH CH2OH DAltrose
Enantiomers of glucose. The enantiomer of lactic Acid. Nomenclature for enantiomers. The absolute configuration of enantiomers can be explained by using: D/L nomenclature system; R/S nomenclature system; D/L system. Although D/L is an old system of nomenclature but still most widely used for amino acids and carbohydrates. In order to determine.

Enantiomers vs Diastereomers What are Enantiomers? Video & Lesson Transcript
The D- and L-glucose are true enantiomers. So, enantiomers, which means that they're complete mirror images. They differ at every single chiral carbon. Now that being said, if the D-aldohexoses, these glucose, if the D- and L-aldohexoses are enantiomers, that means that all of the D-aldohexoses have to be diastereomers of each other, because.

Adisi Nukleofilik
The Epimers of glucose involve some formations, some examples are starch, glycogen, glucose, polysaccharides, and oligosaccharides. The stereoisomers β-D-mannopyranose and β-D-glucopyranose are known as epimers because they differ only in the C-2 position of stereochemistry. The hydroxyl group in the β-D-glucopyranose molecule is equatorial.
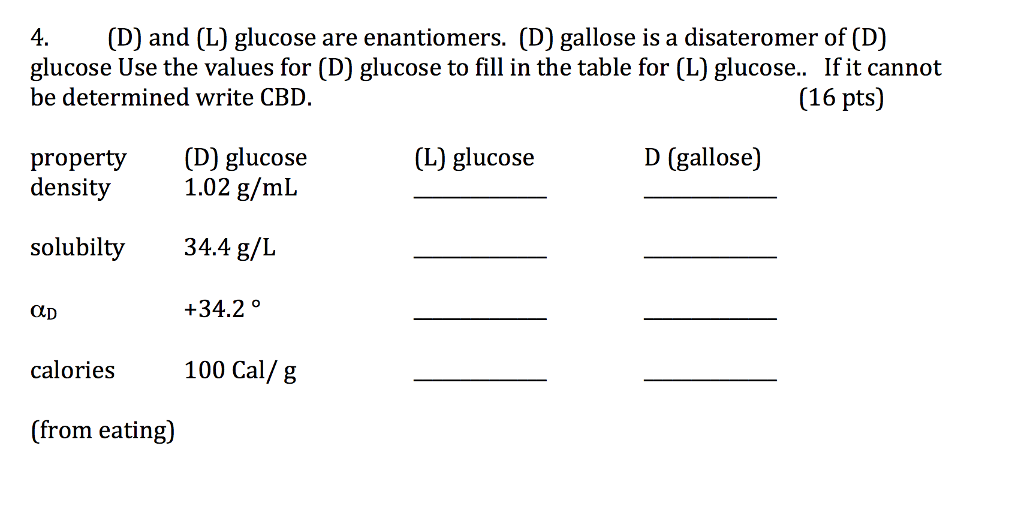
Solved (D) and (L) glucose are enantiomers. (D) gallose is a
The confusion about D and L arises because the L sugars of a given name (glucose, for example) are mirror images of the D sugars of the same name. This concept is most easily seen with glyceraldehyde. In the same way D- and L- glyceraldehyde represent two enantiomers, the D- and L- forms larger monosaccharides are enantiomers of one another.

ɑDglucoses and βDglucose are anomersdiastereomers that differ in only one chiral center D
Q1 What are Epimers with examples? Epimers are carbohydrates that differ in the location of the -OH group in one location. Both D-glucose and D-galactose are the best examples. D-glucose and D-galactose epimers create a single difference at C-4 carbon. They are not enantiomers, they are just epimers, or diastereomers, or isomers. Q2

CH103 Chapter 6 Natural Products and Organic Chemistry Chemistry
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves.

D and LGlucose are enantiomers. Chemistry, Chemistry notes, Science chemistry
Thus, L-glucose and D-glucose are enantiomers, but D-Erythrose and D-Threose are diastereomers. Figure \(\PageIndex{1}\): Diastereomers. Figure \(\PageIndex{2}\): Enantiomers. Sugars of 5-7 carbons can fairly easily form ring structures (called Haworth structures). For aldoses like glucose, this involves formation of a hemi-acetal.
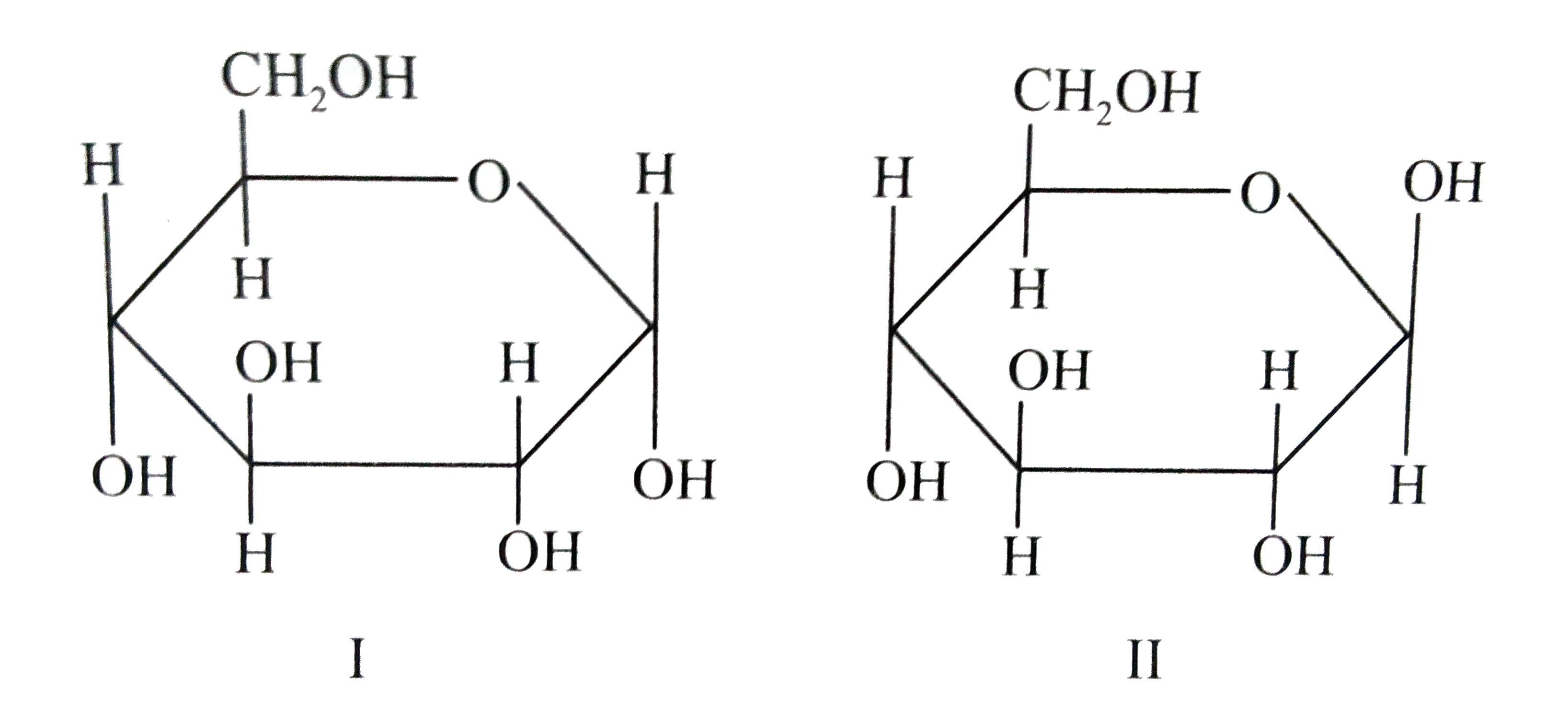
Alpha D Glucose and Beta D Glucose Are Enantiomers
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves.
:max_bytes(150000):strip_icc()/chirality-diagram-56a12ca45f9b58b7d0bcc7b5.png)
What is an Enantiomer?
1 Answer Maxwell Aug 12, 2016 They are not enantiomers. They are diastereomers. Explanation: Diastereomers are molecules that have 2 or more stereogenic centers and differ at some of these centers with respect to absolute configurations. This disqualifies them from being mirror images of each other.
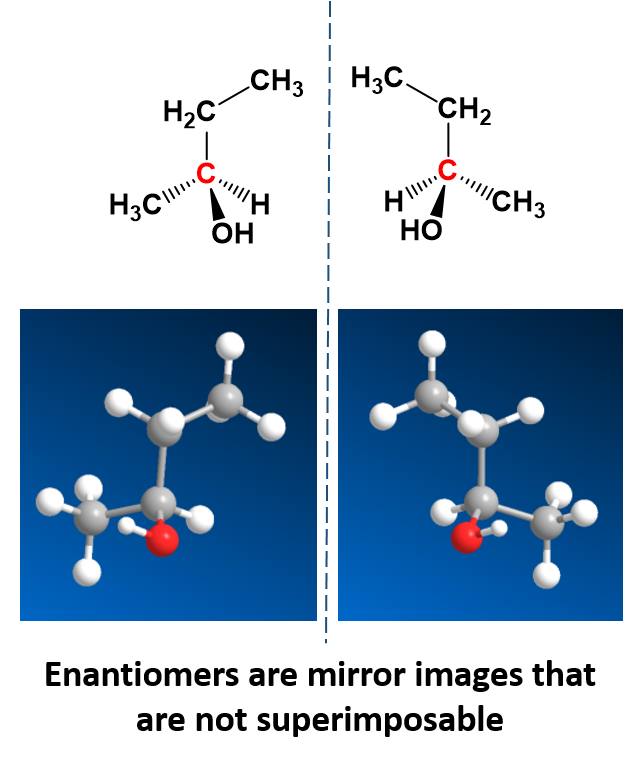
CH105 Chapter 5 Introduction to Organic Chemistry Chemistry
The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves 14 diastereomers of D-glucose: these are molecules in which at least.
Chiral responsive CdotsAu NP complex towards glucose enantiomers. (a)... Download Scientific
Their enantiomers were given the same name with the introduction of systematic nomenclatures, taking into account absolute stereochemistry (e.g. Fischer nomenclature, d / l nomenclature). For the discovery of the metabolism of glucose Otto Meyerhof received the Nobel Prize in Physiology or Medicine in 1922. [16]

Enantiomers in nature CheMystery
Therefore, D and L Glucose are enantiomers, while ɑ-D-glucose and β-D-glucose are diastereomers. To summarize what we learned about epimers and anomers Epimers are diastereomers that differ in the configuration of only one chiral center. Anomers are epimers specifically applied to characterize cyclic carbohydrates.
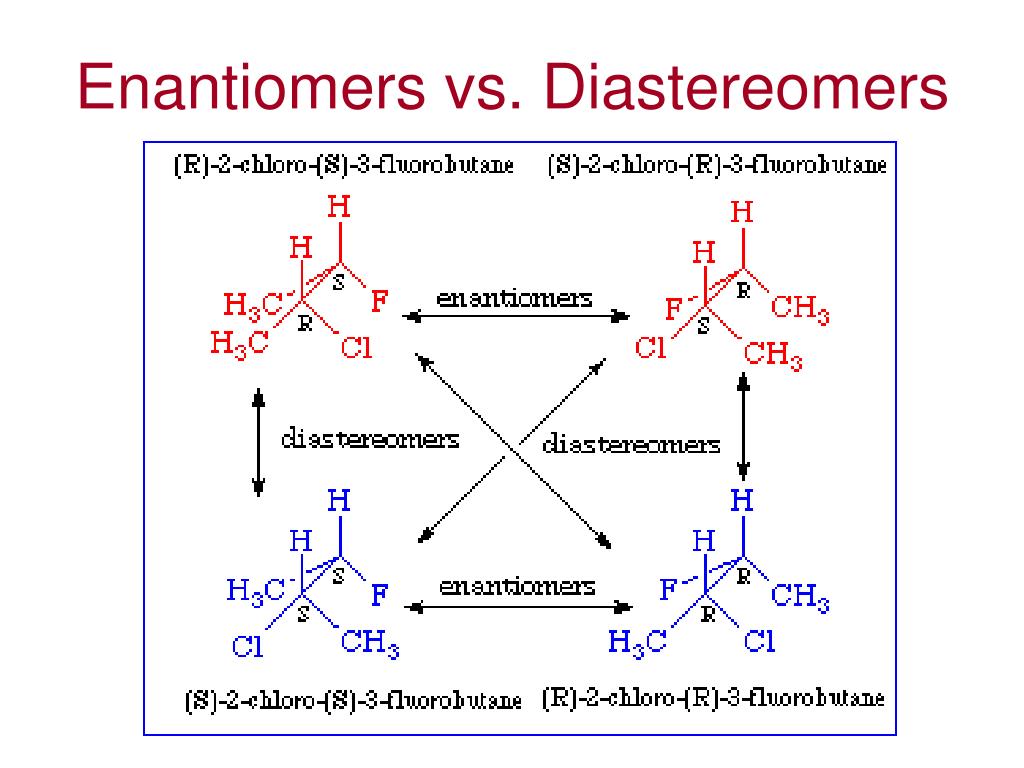
PPT Isomerism Recap PowerPoint Presentation, free download ID397852
Although sugar enantiomers may display the same or similar biological activity, it is obvious that the protein-binding properties of a d -sugar component of a lead synthetic glycoside are different from its L-enantiomer (or vice versa).
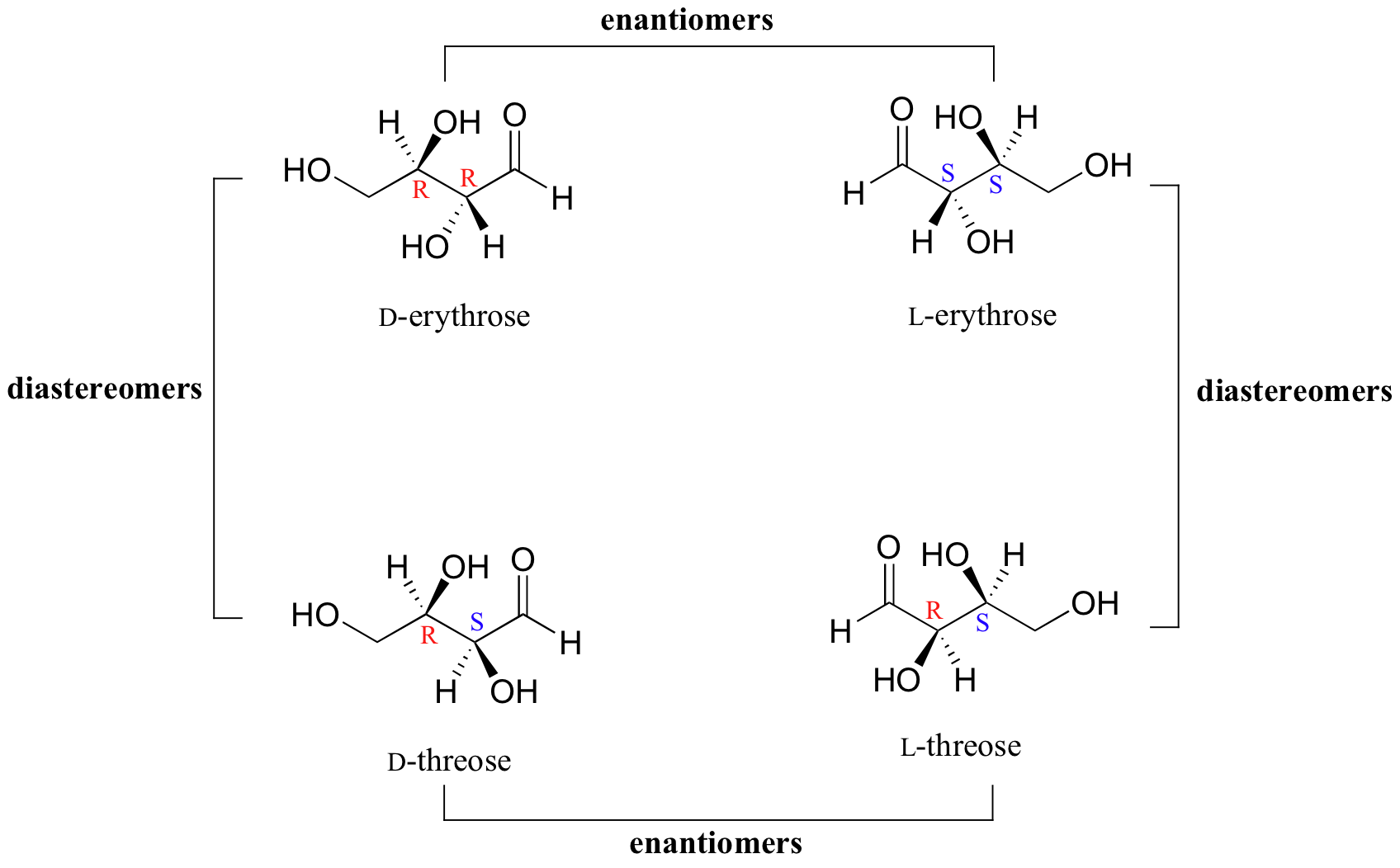
Chirality and Stereoisomers Chemistry LibreTexts
There are three common naming conventions for specifying one of the two enantiomers (the absolute configuration) of a given chiral molecule: the R/S system is based on the geometry of the molecule; the (+)- and (−)- system (also written using the obsolete equivalents d- and l-) is based on its optical rotation properties; and the D/L system is based on the molecule's relationship to.